Research - Journal of Molecular Pathophysiology (2023)
Effect of Antimicrobial Peptides and Chemicals Produced by Animals on Agrobacterium rhizogenes
Al-Baraa Akram El-Sayed*Al-Baraa Akram El-Sayed, Department of Pharmaceutical Biotechnology, De Montfort University, Leicester, United Kingdom, Email: albraaakram94@gmail.com
Received: 26-Apr-2023, Manuscript No. JMOLPAT-23-97023; Editor assigned: 28-Apr-2023, Pre QC No. JMOLPAT-23-97023 (PQ); Reviewed: 15-May-2023, QC No. JMOLPAT-23-97023; Revised: 22-May-2023, Manuscript No. JMOLPAT-23-97023 (R); Published: 29-May-2023
Abstract
Agrobacterium rhizogens is the former name of Rhizobium rhizogens which is found mostly in soil and causes hairy root disease in the dicotyledonous plants. It induces the formation of proliferative multiple branched adventitious roots at the sites of infection, which is called hairy roots disease which are not yet resolved.
Generally, the following steps can be used to separate the genetic transformation: In the first step, Agrobacterium rhizogens detects plant roots emit phenolic chemicals, which encourage the bacteria to adhere to the root. The second stage involves converting the t-DNA into Agrobacterium rhizogens cells, where T complexes are created. In the third phase, the type IV protein secretion system is used to transport T-complexes from the bacterium to the host plant’s genome. On the t-DNA of Agrobacterium rhizogens, there are two different sequences called the TL and TR borders. TL-DNA and TR-DNA typically travel and integrate into the host plant genome separately; but the TL only is essential and enough to cause bushy roots. The sequence analysis of TL-DNA revealed four open reading frames-rolA, B, C, and D, that are necessary for induction of the hairy root. The RolB gene appears to be the most effective in producing hairy roots since a loss of function mutation at this locus makes the plasmid non-virulent.
In addition to the ban on using antibiotics as growth promoters in feed, the issue of multidrug-resistant diseases like bacteria, fungus, and yeast has drawn attention to the need for finding substitutes for conventional antibiotics, such as natural compounds with antibacterial action. Research has shown that natural antimicrobial peptides and chemicals derived from animal secretions and some insect venom have antimicrobial activity against pathogens with lower resistance and higher synergistic effects if given in combination with blends of them. This has attracted a lot of interest.
The optical density analysis approach will be used in this study to explore antimicrobial compounds collected from giraffes as well as their activity against the bacterium Agrobacterium rhizogens. Then, using applications like Gene5, GraphPad Prism, and Clone Manager, their Minimum Inhibitory Concentrations (MIC) and ICs 50 will be established. Additionally, some chemicals found in animal secretions will be tested for their antimicrobial activity.
Keywords
Agrobacterium rhizogens, Plant cells, DNA, Antibacterial, Fagopyrum tataricum
Introduction
Agrobacterium is a gram-negative, aerobic, rodshaped bacterium that is a member of the Rhizobiaceae family; it is infective to plant cells and opportunistic to human with low immunity only. It is grown on YMB which is yeast mannitol broth [1, 2]. Culturing techniques require the optimal temperature for growth of the room temperature. Colony morphology shows convex, circular and smooth characteristics, while colour will be non-pigmented. The etymological origin of the name of species rhizogens comes from the Greek words rhiza meaning a root and gennao to make, thus resulting in a root-producing bacteria.
The rhizogens was first isolated in 1930 and it is found commonly in leguminous plants. It is known for its ability to induce tumours in the hairy roots. This is characterized by the overabundant growth of the plant’s root system. The ability of hairy root plants to expand rapidly in the absence of exogenous plant growth regulators is one of their fundamental traits as shown in Figure 1.
Figure 1. Carrot slices infected by Agrobacterium bacteria show cellular calli (green) from which roots arise. This strain does not integrate any IPT gene (responsible for the synthesis of cytokinins) into the genome of plant cells. An infected cell can therefore only mass produce auxin. Consequently, the AIA/CK hormonal balance leans in favor of auxin, hence rhizogenesis.
Agrobacterium rhizogens is also used successfully to transform tea. Some workers used Agrobacterium rhizogens strains like A4 and MTCC532 to transform tea leaves. The obtained frequency of transformation (70%) was quite encouraging. The transformation work was effective at a concentration of 60 mg/L. In order to successfully create callus and hairy root cultures, tissue culture was carried out in medium supplemented with 30 mg/L of maltose. It was discovered that the hairy roots had a significant ability for diversification, dense white fibrils, and quick growth in the face of geotropism. Several cultivars were discovered to have differing capacities for callus development. Hairy roots have been used for many purposes over the last 40 years, ranging from recombinant protein production and metabolic engineering to analysis of rhizomes’ physiology and biochemistry. Recently, Agrobacterium rhizogens mediated hairy root production has been used as a biotechnology tool in many of plant species to discover new biological approaches. Metabolic enzyme function can be determined by gene overexpression or RNA interference insights with the aid of hairy root transformation, for example the elucidation of an enzyme that functions in presence of pyridine alkaloid biosynthesis in Nicotiana glauca. Stable isotope studies can also be done in presence of hairy roots to distinct reactions within biosynthetic pathways. For example, studies on hairy roots of Ophiorrhiza pumila showed that camptothecin is derived from the 2-C-methyl-D-erythritol-4-phosphate as well as shikimate pathways. Agrobacterium rhizogens was used to identify plant genes which are able to suppress programmed cell death triggered in plants by transformation of individual members of complementary DNA (c-DNA) library in to tomato roots. In addition to that, Agrobacterium rhizogens transformed roots of tomato expressing the baculovirus which demonstrated its presence of plant of proteases with substrate site specificity that is functionally equivalent to animal caspases. Agrobacterium rhizogens can be also used as a tool to determine spatial and temporal aspects of gene expression in plants for the sake of interference of signalling pathways associated with pathogen response. A group of genes were tested in tomato and potato for their responsibility in the production of a nematode feeding structure during infection by nematode cysts. Additional tools were used to enable the hairy roots transformation efficiency include plasmid of pHairy that uses the red fluorescence of protein DsRed2 for visual selection of hairy roots transgenicity in soybean. This reporter greatly increased the detection percentage transformants and was used as a tool to detect the exact function of soybean Nod factor reporter.
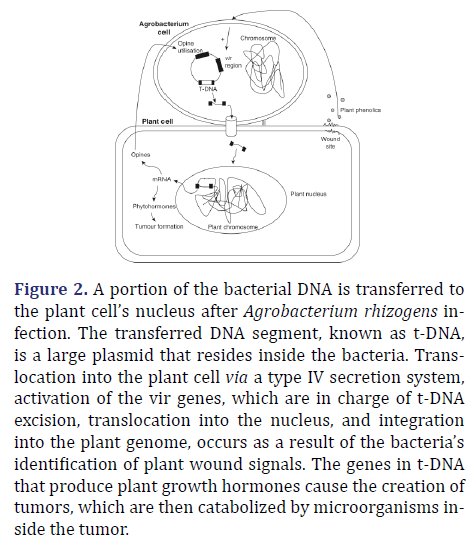
As a natural genetic engineer, Agrobacterium rhizogens has attracted interest in transferring genes to plants since the middle of the 1980s. Plants become infected and develop hairy roots as a result of the insertion of the RI (root inducing) plasmid of Agrobacterium rhizogens’ t-DNA region into the plant’s DNA. Frequent branching and a high density of capillary roots are two traits of hairy roots, a high capacity for biomass generation, and quick development. The altered root tissue can continue to grow for years if the bacteria-induced roots are kept in-vitro in a nutrient medium. For many hundred species, these civilizations have been in place. Many of these plants, especially those from the Solanaceae, Asteraceae, and other families, contain secondary metabolites like alkaloids in their roots that are important for pharmacology. Due to its quick start and rapid growth, hairy root culture is a large potential commercial source of significant root borne compounds as shown in Figure 2.
Figure 2. A portion of the bacterial DNA is transferred to the plant cell’s nucleus after Agrobacterium rhizogens infection. The transferred DNA segment, known as t-DNA, is a large plasmid that resides inside the bacteria. Translocation into the plant cell via a type IV secretion system, activation of the vir genes, which are in charge of t-DNA excision, translocation into the nucleus, and integration into the plant genome, occurs as a result of the bacteria’s identification of plant wound signals. The genes in t-DNA that produce plant growth hormones cause the creation of tumors, which are then catabolized by microorganisms inside the tumor
Agrobacterium rhizogens produces t-DNA, a tumor-inducing DNA fragment with the innate capacity to transfer to and integrate with the genome of the host plant. Integration of t-DNA in the genome of the host leads to random insertion of mutagenesis has a few numbers of copies but inheritance is stable. The phenotype of tumour induction is closely related to the presence of large tumour inducing plasmid Ti-plasmid, containing t-DNA, to introduce its DNA plasmid into host plant cells. Because of this it has been used for a variety of purposes e.g. production of plant endangered species, production of recombinant protein and genetic engineering.
Because the transformation and regeneration protocols are more standardized, mutant t-DNA lines are widely available around the world. For example, more than 45000 flanking sequence tags have been developed so far using these lines. These mutants are very large helpful to characterize gene function. Another key gene involved in tissue culture at the lamina joint leaf, were identified via t-DNA mutant screening.
To promote rapid development without requiring any auxins from external sources, hairy roots are preferred. For incubation, light is typically not required. Metabolite production is stable, and so is genetic stability. Due of all these benefits, hairy root culture technique has been used to manufacture a variety of root-derived plant products that were previously deemed unsuitable for cell culture.
Although several studies have shown that plant cell culture is not always effective at producing them, undifferentiated plant cell cultures are effective at developing significant secondary metabolites. Even if the necessary chemicals were created by the cultivated cells, their concentration was frequently much lower than that of entire plants.
Plant roots accumulate a number of secondary metabolites with potential for use in pharmaceuticals. Hairy root cultures, as opposed to undifferentiated cell cultures, can frequently produce the same compounds as the roots of the entire plant. The ability of hairy root cultures to create secondary metabolites, including tropane from Atropa belladonna and scopolia as well as nicotine from Nicotiana rustica, was demonstrated in three different laboratories in 1986.
Ecology and pathology
The mechanism by which Agrobacterium infects wounded plants starts by the attraction of the bacterial cells to the sites of wound due to the production of phenolic compounds by chemo taxis. Once the bacteria reach the wound site, they infect the plant by integrating their pathogenic Ri plasmid into the plant genome which leads to the development of hairy root disease.
Under normal conditions, Agrobacterium is restricted to a limited number of plant species such as apple, cucumber, tomato and melon. However, under laboratory conditions, more than 450 plant species are susceptible to infection by Agrobacterium. One of the key methods by which researches can differentiate and determine Agrobacterium from other species within the same genus of these bacteria is the infection polarity which is possibly caused by the presence of a second t-DNA which encodes genes that produce auxins in plants transformed by the agropine type Ri plasmid. Strains that can complete with Agrobacterium such as agrocin producing strains are capable of inhibiting infection by these species of bacteria.
Species that produce antibacterial compounds: A trustworthy supply of nutrients like metal and metallic components is the chicken egg. The chemical, physical, and defense systems of the egg guard against invasion, infection, and multiplication for the embryo. The egg shell physically prevents microbial penetration of the egg wall, and as a result, the egg shell membrane does as well.
The pH scale and uniformity prevent bacteria multiplication and proliferation chemically. In terms of biology, the albumen contains a variety of proteins with antimicrobial properties, such as the ability to lyse microbial cells and to bind metals. These properties are forms of the egg’s natural and innate psychoanalytic process. The various biological roles of the egg components, along with their immunomodulating, antibacterial, and peptidase-inhibiting properties, may all emphasize the significance of those compounds in preventing and defending both humans and animals against disease [3].
Another illustration is the hoopoe preen, which secretes antimicrobial compounds that were examined with antibiotic injections to see if the oil gland-dependent bacterium was responsible for their creation. This bird produces two types of chemical secretions that differ in chemical makeup [4]. The difference between them is that one of them has a mixture of volatile compounds and the other does not. The powerful bactericidal properties of all volatile compounds have been demonstrated, and mixtures of those chemicals at a wide range of concentrations have been found to inhibit the growth of some bacterial strains that have been examined in various methods [5].
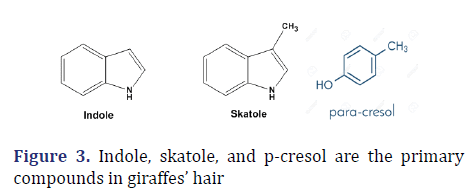
These compounds, which can be recognized in a variety of ways, are produced by giraffes. Gas chromatography/ mass spectroscopy was used to examine hair samples from male and female giraffes that had been treated with methylene chloride or DMSO [6]. This method creates the compounds indole and 3-methyl indole, which are largely responsible for the giraffe’s characteristic odor. P-cresol, heptanal, octanal, nonanal, benzaldehyde, octane, hexadecenoic acid, and tetra-decanoic acid are other chemical extracts as shown in Figure 3.
According to reports, indole and skatole were discovered in the giraffe’s hair and other dermal components. Indole and skatole are extremely odoriferous to humans and require biological process and fungi static activity against a wide variety of microbes that may be present on the skin in order to be used for antimicrobial topical preparations and antimicrobial patches that may be absorbed [7].
The following Table 1 displays several compounds’ whole particle current.
| Compound | Male (%TIC) | Female (%TIC) |
|---|---|---|
| Octane | 5.6 | 6.9 |
| Benzaldehyde | 3.7 | 5.7 |
| Heptanal | 5.3 | 2.3 |
| Octanal | 2.9 | 3.6 |
| Nonanal | 22.5 | 28.9 |
| p-Cresol | 5.5 | 3.0 |
| Indole | 6.5 | 3.0 |
| 3-Methylindole | 14.0 | 4.7 |
| Tetradecanoic acid | 8.1 | 11.4 |
| Hexadecanoic acid | 11.9 | 11.3 |
| 3,5-Androstadien-17-one | 8.1 | 6.9 |
| Minor Compoundsa | 6.0 | 12.5 |
Many compounds obtained from giraffes can stop cutaneous bacterial growth. For instance, penicillin-resistant Staphylococcus aureus is inhibited by indole, benzaldehyde, octanal, p-cresol, Hexadecenoic acid, nonanal, and saturated fatty acids with a high acidity. Methicillin-Resistant Staphylococcus Aureus (MRSA) can be treated with these drugs.
The activity of these compounds is typically moderate, but when they work together synergistically, their activity becomes more impregnable. For instance, the addition of indole to certain chemicals can reduce the Minimum Inhibitory Concentration (MIC) from 1600 g/ml to 6.25 g/ml, which prevents the eubacteria bacterium, which causes caries, from growing.
Uses for animal secretions in medicine: They were created as therapeutic agents for humans due to their broad scope, ease of chemical synthesis, and minimal possibility of microbial resistance [8]. Animals naturally employ peptides to fight germs since they are in conflict with them, which is why animals’ architecture is designed to do so.
Generally, hypoalbuminemia and total p-cresol increase the free fraction of p-cresol; patients hospitalized with infections are detected with high level of free p-cresol. In-vitro, free p-cresol high level contains a negative result on WBC production, thus dangerous nutrition might exacerbate the toxicity of p-cresol [9] however if p-cresol are used locally, this might decrease its toxicity and facet effects.
Multiple drug medical care could be a difficult drawback in medication today that crystal rectifier to drugdrug interactions which are magnified hugely thus it’s involved in drug analysis and development. Some medications were withdrawn from market as terfenadine and mibefradil thanks to drug-drug interactions [10, 11].
Materials and Methods
The ability which Agrobacterium has in introducing its Ri pathogenic plasmid into host plant cells and induce tumour of root system has been studied for the sake of increasing yield of the important metabolites. One study sought to devise a protocol for a successful induction of different strains of Agrobacterium in the plant Fagopyrum tataricum which is commonly known as tartary buckwheat with the target of the highest growth rate determination, number of roots, efficiency of transformation and total content of rutin and anthocyanin. Among the used strains of Agrobacterium, R1000 strain was the most promising candidate as it showed the highest values for the above mentioned desired characteristics. Fagopyrum tataricum has been a goal of latest research because of the presence of pharmacologically important phenolic compounds. The induction protocol consisting in dipping the plant seeds into suspensions of the different strains of Agrobacterium for 20 minutes, followed by drying on a sterile tissue paper and incubation under dark conditions at 25οC on a half strength solid MS medium for 48 hours. After incubation the samples of the hairy root were observed and analysed through PCR analysis. Finally, the amount of the desired phenolic compounds was quantified through HPLC protocols, which isolated these compounds from the hairy root samples. Among the isolated phenolic compounds, rutin was the most available as it contributed with 92% concentration of the total phenolic compounds. Rutin is flavonol compound which has been used for many therapies because of its pharmacological activities including antioxidant, cytoprotective, vasoprotective, anticarcinogenic, neuroprotective and cardioprotectice activities.
A description of MIC testing
In order to perform the minimum inhibitory concentration test, a pure microbe culture had been established in the proper broth. To get a cell concentration of almost 1 million cells per millilitre, the culture has been standardised using accepted standard microbiological techniques. The more standardized the microbiological culture, the more consistent the findings of the tests.
The antibacterial agent was regularly diluted, typically 1:1 with the right diluent. Following antimicrobial dilution, a volume of standard volume equal to the volume of diluted antimicrobial was added to each vessel of dilution until the microbial concentration reached over 500000 cells per ml.
Incubate the serially diluted antimicrobial agent for the appropriate amount of time between 18 and 24 hoursat the correct temperature depending on the kind of bacterium.
Benefits of MIC
• Reproducibility is made possible by ease of preparation and simplicity.
• Is possible to perform on a very small scale without needing a lot of the antimicrobial agent, which is important for antimicrobial compounds used in experimental settings, such as biologically produced antimicrobial peptides.
Drawbacks of MIC
• The apparent findings of the MIC test may be impacted by variations in test parameters. For instance, a long incubation period will result in a higher MIC, whereas a lower inoculum concentration will appear to have a lower MIC.
• Certain microorganisms in bacteriostatic antimicrobial agents will cease growing but not be destroyed; there may still remain the same number of cells awaiting neutralization of the antimicrobial agent.
Preparing a culture of microbes: It mostly depends on incubating each microorganism at the correct temperature and in the appropriate culture medium. You’ll need distilled water, yeast mannitol broth, an empty bottle, a scale, and a spatula.
• Fill an empty bottle with the prepared amount and yeast mannitol broth.
• Bottle of yeast mannitol broth should spend the night being autoclaved at 121 degrees Celsius.
• In mannitol broth made from yeast, Agrobacterium is cultivated.
• Get Petri dishes containing Agrobacterium, prepare a test tube for media, fill it with 10 ml of the culture, and add the test tube.
• Prepare Agrobacterium culture media by doing the following:
• Identify the microorganism and culture media on the bottle’s label.
• Take a wire loop, sterilize it by holding it in the Bunsen burner flame until it glows red throughout its length, and then remove it to cool.
• After picking up the petri dish and opening the lid to create a streak, the lid of the culture media tube was opened, and the loop was dipped into the bottle.
• Each one should spend the night being incubated at the proper temperature.
• By altering the ratios between the bacterial culture and broth, the culture had been thinned as shown in Table 2.
| Name of M. O | Ratio | Culture (ml) | Broth (ml) |
|---|---|---|---|
| Agrobacterium | 1:4 | 5 | 15 |
Analysing optical density: The solvent is merely added to tare the device in a clean tube. An aseptic 1/20 culture is created from 10 ml of the tube by mixing 50 l of the culture with 950 l of T.E. buffer (tris and EDTA). The final optical density was calculated by multiplying the result by 20.
If optical density was this great, a 1/40 ratio representing 25 l of culture combined with 975 l of T.E. could have been created.
Preparing chemicals: Using DMSO as a solvent, indole, methyl indole (skatole), and p-cresol are extracted.
Each individual microorganism required four of the 24 well plates, which were divided as shown in Tables 3 and 4.
| 1 | 2 | 3 | 4 | 5 | 6 | |
| A | 1 | 2 | 3 | 4 | 5 | 6 |
| B | 1 | 2 | 3 | 4 | 5 | 6 |
| C | 7 | 8 | 9 (+) | 10 (-) | Empty | Empty |
| D | 7 | 8 | 9 (+) | 10 (-) | Empty | Empty |
| Code | Well number | Ratios | Concentrations |
|---|---|---|---|
| 1 | A1 and B1 | 1 | 20 mM |
| 2 | A2 and B2 | ½ | 10 mM |
| 3 | A3 and B3 | ½ | 5 mM |
| 4 | A4 and B4 | 1/5 | 1 mM |
| 5 | A5 and B5 | ½ | 500 µM |
| 6 | A6 and B6 | ½ | 250 µM |
| 7 | C1 and D1 | 1/10 | 25 µM |
| 8 | C2 and D2 | ½ | 12.5 µM |
| 9 | C3 and D3 | Positive control | just Cells |
| 10 | C4 and D4 | Negative control | No cells |
| Empty | C5 and D5 | Empty | Empty |
| Empty | C6 and D6 | Empty | Empty |
With the exception of the final eight wells, add 20 M of indole to 500 M of cells and medium on the first plate. As a positive control, place only cells without chemicals in two wells, and as a negative control, place only media in the remaining two wells.
On the second plate, 500 microliters of cells and medium were combined with 20 microliters of skatole in all except the final eight wells. For the positive and negative controls, the remaining two wells contained only media.
Add 20 M of p-cresol to 500 M of cells and medium in all but the last eight wells of the third plate. Place two wells as a positive control with just chemical-free cells, and the other two wells as a negative control with only medium.
Place 20 M of a mixture of indole, cresol, and skatole in the last eight wells of the fourth plate along with 500 M of cells and medium. For the positive and negative controls, place just the media in the other two wells.
Repeat this procedure for each plate, changing the microorganisms that are used. For each microbe, make four plates and put indole, methyl indole (skatole), and p-cresol into each one. Then, note on each plate the kind of cells and medium, and chemicals that were used in accordance with the table 3. Then, overnight incubate them as shown in Figures 4 and 5, Table 5.
| Code | Well number | Dilutions | Concentrations |
|---|---|---|---|
| 1 | A1 and B1 | 1 | 4000 µM |
| 2 | A2 and B2 | ½ | 2000 µM |
| 3 | A3 and B3 | ½ | 1000 µM |
| 4 | A4 and B4 | 1/5 | 200 µM |
| 5 | A5 and B5 | ½ | 100 µM |
| 6 | A6 and B6 | ½ | 50 µM |
| 7 | C1 and D1 | ½ | 25 µM |
| 8 | C2 and D2 | ½ | 12.5 µM |
| 9 | C3 and D3 | 1/10 | 1.25 µM |
| 10 | C4 and D4 | Positive control | Chemicals |
| Empty | C5 and D5 | Positive control | Chemicals |
| Empty | C6 and D6 | Negative control | Broth only |
Chemical optical density analysis: Enter the data into excel sheets that provide well concentrations, microbe names, and chemical names. To obtain data that demonstrate the optical density using fluorescence assays of each well that assess the impact of each chemical and the response of the microorganism to it, enter each plate separately into the Gen5 program. Optical density is preferred over ultraviolet spectrometry since the UV wave itself had an antibacterial activity that could interfere with the effects of chemical action on microorganisms. Using the computation:
%of potency=100-(O.D of chemical/O.D of Blend)*100
Analysis of statistics for microorganisms: When you had more optical density, you would need to reduce concentration, so the lower IC is obtained by using GraphPad prism to enter the data obtained from the excel sheet of gene 5 server and then making non-linear regression to obtain graph showing the growth inhibition of each microorganism as we see it. This curve can be fitted by dose response inhibition and then drawing log inhibitor by μM versus response to obtain variable slopes consists of four parameters.
Results
Optical density analysis as shown in Table 6
| Reading | Optical density | |
|---|---|---|
| Blank | Zero | Zero |
| Agrobacterium | 0.01 | 0.2 |
Chemical synthesis and optical density analysis based on chemicals
It depends on the types and species of microorganisms as well as the types and concentrations of chemicals when computing optical densities for results connected to each concentration, including positive and negative controls.
Agrobacterium
Blend: It is demonstrating that it killed cells at all concentrations, ranging from 4 mM to 1.25 M, indicating that the MIC is lower as shown in Figure 6 and Table 7.
| Concentration | Mean | ||||
|---|---|---|---|---|---|
| 4 mM | 0.094 | 0.09 | 0.092 | ||
| 2 mM | 0.092 | 0.086 | 0.089 | ||
| 1 mM | 0.109 | 0.113 | 0.111 | ||
| 200 µM | 0.112 | 0.127 | 0.1195 | ||
| 100 µM | 0.114 | 0.121 | 0.1175 | ||
| 50 µM | 0.129 | 0.141 | 0.135 | ||
| 25 µM | 0.113 | 0.12 | 0.1165 | ||
| 12.5 µM | 0.12 | 0.12 | 0.12 | ||
| 1.25 µM | 0.18 | 0.141 | 0.1605 | ||
| Positive controls | 0.144 | 0.143 | 0.151 | 0.154 | 0.148 |
| Negative controls | 0.111 | 0.154 | 0.1325 |
Cresol: It is demonstrating that it killed cells at all concentrations, ranging from 4 mM to 1.25 μM, indicating that the MIC is lower as shown in Figure 7 and Table 8.
| Concentration | Mean | ||||
|---|---|---|---|---|---|
| 4 mM | 0.16 | 0.118 | 0.139 | ||
| 2 mM | 0.129 | 0.125 | 0.127 | ||
| 1 mM | 0.23 | 0.12 | 0.175 | ||
| 200 µM | 0.216 | 0.155 | 0.1855 | ||
| 100 µM | 0.225 | 0.147 | 0.186 | ||
| 50 µM | 0.327 | 0.129 | 0.228 | ||
| 25 µM | 0.295 | 0.123 | 0.209 | ||
| 12.5 µM | 0.26 | 0.137 | 0.1985 | ||
| 1.25 µM | 0.183 | 0.119 | 0.151 | ||
| Positive controls | 0.289 | 0.125 | 0.158 | 0.119 | 0.17275 |
| Negative controls | 0.137 | 0.104 | 0.1205 |
Indole: It is showing that it killed cells in all concentrations from 4 mM until the concentration of 1.25 μM which means that the MIC is lower as shown in Figure 8 and Table 9.
| Concentration | Mean | ||||
|---|---|---|---|---|---|
| 4 mM | 0.089 | 0.09 | 0.0895 | ||
| 2 mM | 0.106 | 0.107 | 0.1065 | ||
| 1 mM | 0.145 | 0.115 | 0.13 | ||
| 200 µM | 0.124 | 0.13 | 0.127 | ||
| 100 µM | 0.133 | 0.145 | 0.139 | ||
| 50 µM | 0.123 | 0.138 | 0.1305 | ||
| 25 µM | 0.138 | 0.129 | 0.1335 | ||
| 12.5 µM | 0.139 | 0.126 | 0.1325 | ||
| 1.25 µM | 0.124 | 0.145 | 0.1345 | ||
| Positive controls | 0.145 | 0.147 | 0.152 | 0.149 | 0.14825 |
| Negative controls | 0.077 | 0.078 | 0.0775 |
Skatole: It is demonstrating that it killed cells at all concentrations, ranging from 4 mM to 1.25 μM, indicating that the MIC is lower as shown in Figure 9 and Table 10.
| Concentration | Mean | ||||
|---|---|---|---|---|---|
| 4 mM | 0.097 | 0.092 | 0.0945 | ||
| 2 mM | 0.085 | 0.085 | 0.085 | ||
| 1 mM | 0.111 | 0.134 | 0.1225 | ||
| 200 µM | 0.12 | 0.124 | 0.122 | ||
| 100 µM | 0.12 | 0.129 | 0.1245 | ||
| 50 µM | 0.118 | 0.129 | 0.1235 | ||
| 25 µM | 0.117 | 0.12 | 0.1185 | ||
| 12.5 µM | 0.126 | 0.12 | 0.123 | ||
| 1.25 µM | 0.128 | 0.136 | 0.132 | ||
| Positive controls | 0.131 | 0.14 | 0.142 | 0.152 | 0.14125 |
| Negative controls | 0.113 | 0.076 | 0.0945 |
Statistical analysis
You can measure the potency in accordance with the values from the preceding tables by looking at graphs that show the inhibition of microorganism growth by plotting an OD 600 by nm value against a chemical’s logarithm by M value in order to determine the inhibitory concentration IC50 and R square.
Agrobacterium
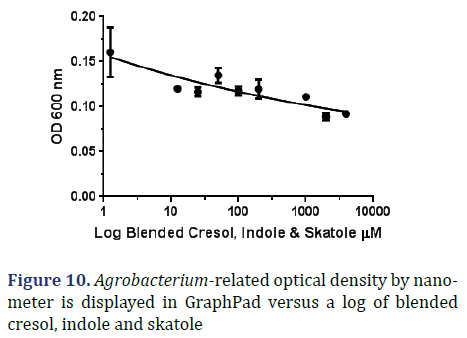
Blend: According to the graph below, which plots OD 600 vs. log skatole concentration, the IC50 is equal to 0 μM and the R square is 0.706 μM as shown in Figure 10.
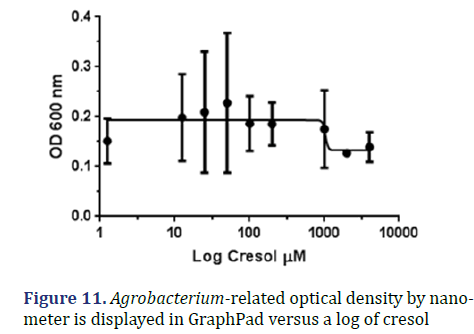
Cresol: The graph below displays the relationship between OD 600 and log cresol concentration; as can be observed, the IC50 is roughly 1035 μM, and the R square is 0.147 μM as shown in Figure 11.
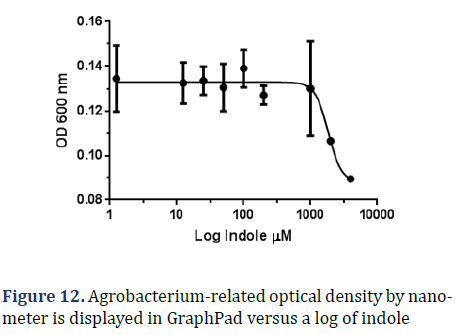
Indole : The graph below compares OD 600 to log indole concentration; as can be observed, the average IC50 is between 1280 μM and 2658 μM, with an IC50 of around 1844 μM and a R square of 0.7747 μM as shown in Figure 12.
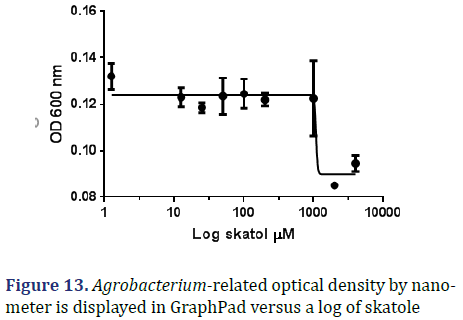
Skatole: The graph below displays the relationship between OD 600 and log skatole concentration; as can be observed, the IC50 is roughly 1082 μM, and the R square is 0.8311 μM as shown in Figure 13.
Discussion
Although it was anticipated to extract compounds from their animal origins, the chemicals had to be purchased because they were expensive. As the optical density of the fungus was abnormal due to clumping and the turbidity is insufficient indication that cells have developed, viable counts and serial dilution procedures can be employed instead.
Agrobacterium
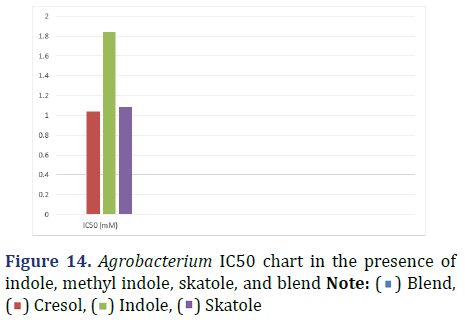
During its chemical preparation it did not show exact results of MIC but when using graph pad it showed an effect with indole only at concentration of 1844 μM which equals 1.844 mM as shown in Figure 14.
Agrobacterium rhizogens can also be used to convert chickpeas. In order to quickly overexpress and knock off genes in roots, for reverse genetic studies in chickpea, root transformation using Agrobacterium rhizogens has become a significant substitute.
Given that the regenerated roots are said to derive from a single cell lineage and can be maintained for an extended period of time under controlled circumstances, allowing for sufficient time for functionally in-depth studies, it is expected that they are non-chimeric. By micro-grafting a composite chickpea plant that blends native type roots and transgenic roots, one can develop an appropriate system for root biology research, functional studies involving diverse microorganisms, and necessary as well as root-pathogen interactome studies.
Composite chickpea plants will be useful in the future for clarifying the biology of dreaded soil-borne illnesses like wilt, dry root rot, different nematodes, parasites of weedy roots, as well as bacteria connected to symbiotic nitrogen fixation in chickpeas and a wide range of abiotic stressors.
The root cell DNA had Ri plasmid genes added, which boosted auxin sensitivity and the growth of lateral roots as a result. Ironically, root initiation is little understood, and in-depth research on that critical process has not yet been done.
Because it is easy to handle, profitable, has a high rate of transformation, and produces roots quickly, Agrobacterium rhizogens is commonly used in gene transfer studies. Secondary metabolite production rates from hairy root cultures can be higher than those of the parent plant. Additionally, it enables genetic alterations to be made in the synthesis’s metabolic process.
Techniques for immobilizing cell culture include hairy root cultures. In order to initiate the entrapped cells’ production of secondary metabolites, elicitor is also administered to the hairy root cultures. Although little is known about the metabolic pathways of secondary metabolism in hairy roots, the most common elicitors, such as jasmonic acid and methyl jasmonate, can be grown to increase metabolite production. Because they are more readily available, less expensive, and easier to supply than compounds of jasmonic acid, sodium chloride, calcium chloride, and potassium chloride can also be employed as elicitors. For instance, ajmalicine production was increased in Rauwlfia serpentina hairy root cultures after seven days of treatment by about 15 times at a 100 mM concentration of sodium chloride. In several Datura species, potassium chloride and calcium chloride were also utilized as elicitors. The highest amounts of hyoscyamine were 2.32, 1.99, and 1.85 fold the control levels for Datura stramonium, Datura tatula, and Datura innoxia hairy root cultures, respectively, with potassium chloride elicitation and 2.08, 2.07, and 1.85 fold the control levels, respectively, with calcium chloride elicitation.
Agrobacterium rhizogens forms part of the bacteria domain as its characteristics follows the category of alphaproteobacteria. Within the genus of Agrobacterium, all the genome shotgun of Agrobacterium rhizogens strain was performed in 2014. The shotgun sequencing approach consists of cutting DNA into smaller fragments which are sequenced individually. Then the approach uses high quality and semi-automated sequencing to formulate a genetic map based on the overlap of the species of Agrobacterium rhizogens the fragments [12]. The obtained data for the Agrobacterium rhizogens strain ATCC 15834 genome map showed that it contained 6668 genes. The sequencing of the species of Agrobacterium rhizogens was performed due to its emerging importance in the fields of genetic engineering and metabolite production for medicinal purposes.
The researchers who conducted the project obtained the ATCC from a hairy root culture work with tomato. By following the DNA extraction and sequencing protocols, the researchers extracted the data that showed the length of the Agrobacterium rhizogens genome, which contains 7070307 base pairs in 43 scaffolds with a GC content of 60% [13]. Annotated method which was held by researchers was used to make confirmation of the species of 54 other 16 sequences and construct a phylogenic tree with the software of FastTree 2 and the result was that the whole genome shotgun project was deposited at the GenBank under the accession number of JFZP00000000.
Another study sought the use of root-inducing phenotype of pathogenic Agrobacterium rhizogens to enhance the growth rate of species of Indian berry [14]. The importance of Indian berry in it medicinal applications as some of it metabolites have shown anticancer activity and treatment of various diseases such as diabetes and fever resulted from malaria. The two Agrobacterium rhizogens strains utilized in the study, MTCC 532 and 2364 were acquired from IMTECH (institute of microbial technology) in India. In this experiment, the researchers infected the plant leaves in order to observe the induction of hairy roots and calculate the metabolite yield. The goal of this study was to devise a protocol for the infection of plants with Agrobacterium rhizogens to increase the production of anticancer compounds and to decrease the overharvesting of this species from its natural habitat [15].
Conclusion
Applying a blend of indole, skatole, and cresol topically to chemicals helps protect the body from dermatological conditions. You can topically apply patches, ointments, or creams produced from this mixture, particularly for certain dermatologic conditions.
Profiling gene expression, chromatin modifications and other molecular signatures at cell type resolution is of great interests which identify events that determine cell type and species specific differences. This can be done by using varieties of techniques such as isolation of nuclei tagged within a specific cell type and translation with purification the affinity of the ribosome which enable the interrogate of multiple stages of gene regulation with one step purification of nuclei and mRNA ribosome complexes respectively. Both intact and translating ribosome affinity purification can take advantage of promoters which are expressed in a specific type of tissues, region or cell type. Once the gene regular localization is detected, it becomes important to know the functional significance of expression in the individual cell type. More recently, RNA guided genome editing which is known as Cluster Regularly Interspaced Short Palindromic Repeats (CRISPR) associated nuclease9 (cas9) was established as a method for targeted mutation of plant genes. In the future, testing this technology as a method for genome editing will enable rapid functional genomic studies to detect the gene function at the cell/tissue specific level.
References
- Uchino Y, Yokota A, Sugiyama J. Phylogenetic position of the marine subdivision of Agrobacterium species based on 16S rRNA sequence analysis. J Gen Appl Microbiol 1997; 43(4):243-247.
- Uchino Y, Hirata A, Yokota A, Sugiyama J. Reclassification of marine Agrobacterium species: Proposals of Stappia stellulata gen. nov., comb. nov., Stappia aggregata sp. nov., nom. rev., Ruegeria atlantica gen. nov., comb. nov., Ruegeria gelatinovora comb. nov., Ruegeria algicola comb. nov., and Ahrensia kieliense gen. nov., sp. nov., nom. rev. J Gen Appl Microbiol 1998; 44(3):201-210.
- Rzedzicki J, Stępień-Pyśniak D. Antimicrobial defence mechanisms of chicken eggs and possibilities for their use in protecting human and animal health. Annales Universitatis Mariae Curie-Skłodowska. Sectio DD, Medicina Veterinaria. 2009; 64(2):1-8.
- Morris JA, Khettry A, Seitz EW. Antimicrobial activity of aroma chemicals and essential oils. J Am Oil Chem Soc 1979; 56(5):595-603.
[Crossref][Google Scholar] [PubMed]
- S. Inouye, H Goi, K Miyauchi, S Muraki, M Ogihara, Y Iwanami. Inhibitory effect of volatile constituents of plants on the proliferation of bacteria: Antibacterial activity of plant volatiles. J-Global 1983;11(11):609-615
- Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother 1972; 2(1):23-28.
[Crossref][Google Scholar] [PubMed]
- Hogan JS, Pankey JW, Duthie AH. Growth inhibition of mastitis pathogens by long-chain fatty acids. J Dairy Sci 1987; 70(5):927-934.
[Crossref][Google Scholar] [PubMed]
- Waugh RJ, Bowie JH. (1997) Antimicrobial Peptides. Wiley 197-223.
- De Smet R, Van Kaer J, Van Vlem B, De Cubber A, Brunet P, Lameire N, et al. Toxicity of free p-cresol: a prospective and cross-sectional analysis. Clin Chem 2003; 49(3):470-478.
[Crossref][Google Scholar] [PubMed]
- Von Moltke LL, Greenblatt DJ, Duan SX, Harmatz JS, Shader RI. In vitro prediction of the terfenadine‐ketoconazole pharmacokinetic interaction. J Clin Pharmacol 1994; 34(12):1222-1227.
[Crossref][Google Scholar] [PubMed]
- Griffin JP. The withdrawal of mibefradil (Posicor). Adverse Drug React Toxicol Rev 1998; 17(2-3):59-60.
- Weber JL, Myers EW. Human whole-genome shotgun sequencing. Genome Res 1997; 7(5):401-409.
[Crossref][Google Scholar] [PubMed]
- Kajala K, Coil DA, Brady SM. Draft genome sequence of Rhizobium rhizogenes strain ATCC 15834. Genome Announc 2014; 2(5):e01108-14.
[Crossref][Google Scholar] [PubMed]
- Brijwal L, Tamta S. Agrobacterium rhizogenes mediated hairy root induction in endangered Berberis aristata DC. SpringerPlus 2015; 4:443.
[Crossref][Google Scholar] [PubMed]
- Akram A, McCann G. Effect of antimicrobial peptides and chemicals produced by animals on Aspergillus fumigatus. Adv Biosci Biotechnol 2021; 12(6):173-192.
Copyright: © 2023 The Authors. This is an open access article under the terms of the Creative Commons Attribution Non Commercial Share Alike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.













 ) Blend,
(
) Blend,
( ) Cresol, (
) Cresol, ( ) Indole, (
) Indole, ( ) Skatole
) Skatole